Abstract
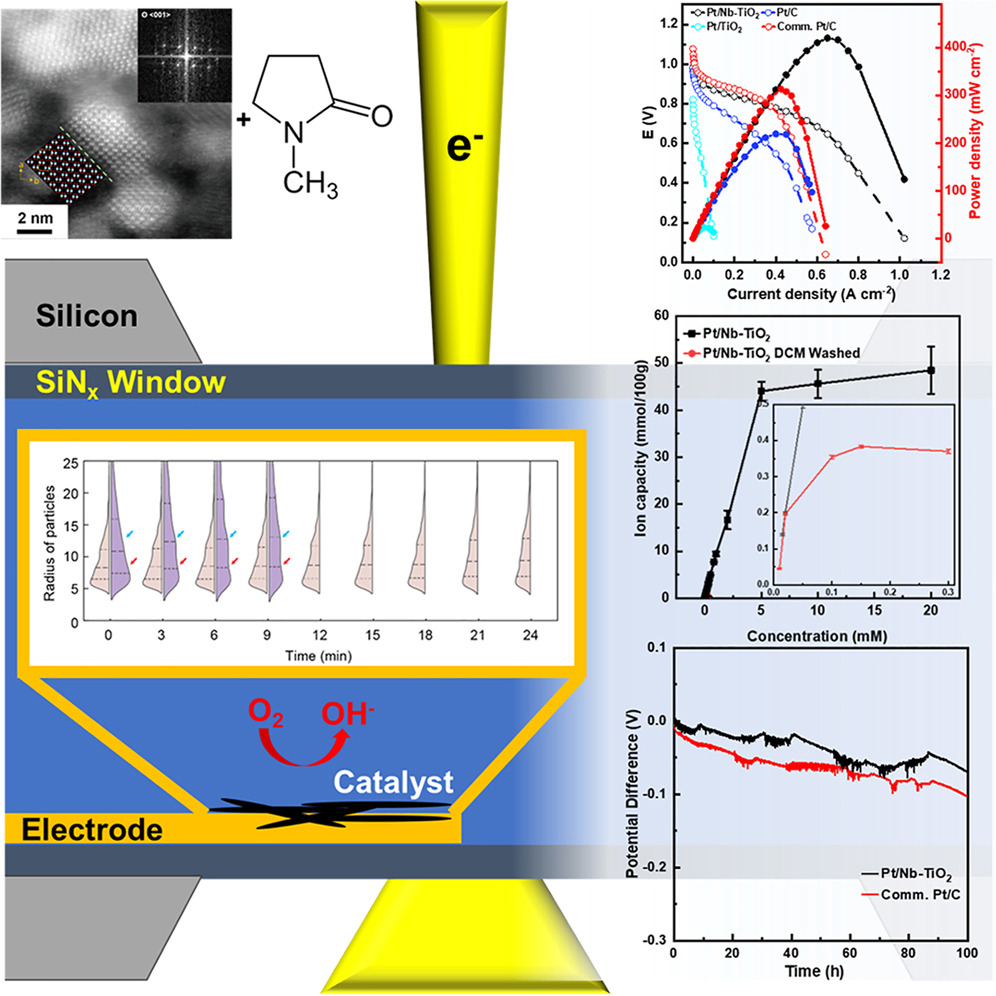
Anion-exchange membrane fuel cells represent a promising and scalable approach for hydrogen energy utilization. However, their development is hindered by the weak bonding between metal catalysts and carbon supports, along with challenges in fabricating electronically/ionically conductive electrodes. Here, we report a composite cathode of Nb-doped brookite TiO2 nanorods that have robust stability when combined with Pt nanoscale catalysts in an alkaline fuel cell. The composite cathode, fabricated without the addition of an ionomer, delivers a power density of 419 mW cm−2 at a current density of 650 mA cm−2 and a voltage retention of 81% at 100 mA cm−2 after 25 h, substantially outperforming a cathode fabricated from commercial Pt/C. Further investigations of the chemical structure, anion exchange capacity, and mass transfer resistance reveal that a solvent residue derived from N-methylpyrrolidone plays an important role in charge transfer and mass transport in the alkaline fuel cell.
Full paper available at: https://www.sciencedirect.com/science/article/pii/S266638642400359X
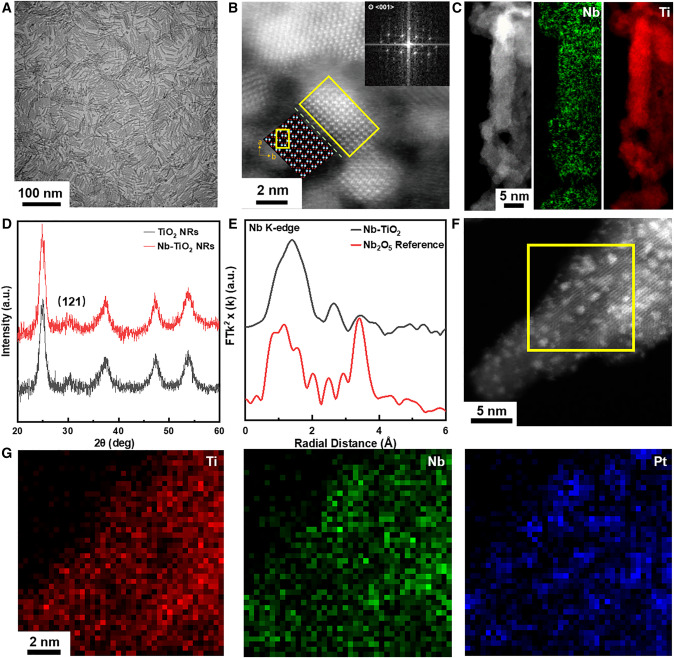
Figure: Morphological and structural characterization of Nb-TiO2 NRs and Pt/Nb-TiO2 NRs
(A) TEM image of Nb-TiO2 NRs with a scale bar of 100 nm.
(B) STEM images of vertically aligned NRs and the atomic model of TiO2, with a scale bar of 2 nm (with the blue and red circles representing Ti and O, respectively), viewed along the <001> direction (left). The inset presents the fast Fourier transform (FFT) image of the Nb-TiO2 NR at the selected area in the center.
(C) EELS elemental mapping images of Nb-TiO2, with a scale bar of 5 nm.
(D) XRD patterns of TiO2 and Nb-TiO2.
(E) Nb K-edge FT-EXAFS spectra of Nb-TiO2 and reference material.
(F) HAADF-STEM image of Pt/Nb-TiO2 with scale bar of 5 nm.
(G) EDS elemental mapping images of Pt/Nb-TiO2 at the selected area, featuring a scale bar of 2 nm.